Background: Chronic myelomonocytic leukemia (CMML) is a clonal hematopoietic stem cell disorder with features of both myeloproliferative neoplasm and myelodysplastic syndrome (MDS). CMML is characterized by persistent blood monocytosis >1 x 109/L, bone marrow dysplasia in one or more hematopoietic cell lines, and increased risk of transformation to acute myeloid leukemia (AML). Our review of SEER Medicare data (Haematologica 2013;98:584) demonstrated that, compared to MDS, CMML has shorter overall survival (OS) and more frequent progression to AML. Hypomethylating agents (HMAs) have become standard therapy for CMML, with reported response rates of 37-69%, but their impact on AML transformation and OS is unclear.
Methods: We retrospectively reviewed CMML patients treated at the University of Maryland Greenebaum Comprehensive Cancer Center between January 2000 and December 2019. Clinical characteristics, treatments, AML progression, time to AML progression (TTP), and OS were recorded and analyzed. Descriptive statistics were used for baseline characteristics and Kaplan-Meier analysis was performed for time-to-event data. Statistical analyses were performed using GraphPad Prism 8®.
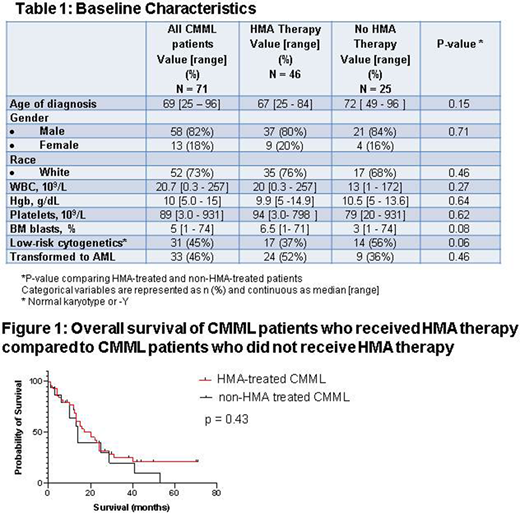
Results: We identified 71 patients with CMML, 82% male and 73% white, with a median age of 69 (range 25 - 96) years; 51% had <10% bone marrow (BM) blasts and 45% had low-risk cytogenetic findings (normal karyotype or -Y). Most patients treated prior to 2005 received hydroxyurea and/or erythropoiesis-stimulating agents or were enrolled on clinical trials, while patients treated since 2005 received HMAs as primary therapy. Median follow-up was 41.1 months. The median OS of the entire cohort was 20 months, with 46% of patients progressing to AML with a median TTP of 11.5 months. By the MD Anderson Prognostic Scoring System at time of diagnosis, CMML was low-risk in 24 patients, intermediate-1 in 16, intermediate-2 in 14, and high-risk in 17.
Forty-six patients received HMAs, with an overall response rate (ORR) of 54% (complete response or partial response), while 25 patients did not receive HMAs. Patient and disease characteristics were similar in HMA- and non-HMA-treated patients (Table 1). The estimated OS of HMA-treated patients was 20 months, compared to 14 months for non-HMA-treated patients (p =0.43) (Figure 1). AML transformation occurred in 52% of patients treated with HMAs, with TTP ranging from 3 to 65 months, and in 33% patients not treated with HMAs, with TTP ranging from 5 to 47 months. Most patients receiving HMAs (63%) received ≥ 6 cycles; 46% transformed to AML despite initial response, often in a sudden and unpredictable manner. HMAs were azacitidine in 13 patients, decitabine in 24, azacitidine followed by decitabine in 4, and decitabine followed by azacitidine in 5. Five CMML patients in our cohort underwent allogenic stem cell transplantation. Four of the five relapsed with transformation to AML post transplant, and only one patient remains in remission, 9 months post transplant.
Conclusions: Despite a 54% ORR, HMA treatment did not have a significant impact on frequency of AML transformation, or OS in our cohort. Based on our data, favorable response rates previously reported with HMAs and also seen in our patients do not appear to translate into decreased frequency of AML transformation or prolonged OS. Though our study is a retrospective study with inherent selection bias, our results underscore the ongoing need for novel therapies and for clinical trials for CMML patients.
Niyongere:Kartos Therapeutics: Other: Received clinical trial research support with Kartos Therapeutics ; Forty Seven: Other: Received clinical trial research support with Forty Seven. Emadi:Amgen: Membership on an entity's Board of Directors or advisory committees; KinaRx: Other: co-founder and scientific advisor; NewLink Genetics: Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Research Funding. Doung:Pfizer: Membership on an entity's Board of Directors or advisory committees, Other: clinical trial research support; Incyte: Other: clinical trial research support; Astex: Other: clinical trial research support; MedPacto: Other: clinical trial research support. Baer:Takeda: Other: Institutional research funding; Oscotec: Other: Institutional research funding; Kite: Other: Institutional research funding; Incyte: Other: Institutional research funding; Forma: Other: Institutional research funding; Astellas: Other: Institutional research funding; AbbVie: Other: Institutional research funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal